Class 16: DNA Sequencing
Bioinformatics
Andrés Aravena
December 06, 2021
Why sequence DNA?
Why do we sequence DNA?
In broad terms there are three things we would like to sequence
- PCR products a.k.a. amplicons
- Random genomic fragments a.k.a shotgun
- RNA messengers
Amplicons
- highly targeted
- enables analysis of genetic variation in specific regions
- uses primers targeted to regions of interest
- useful for the discovery of mutations in complex samples
- such as tumors mixed with germline DNA
- used in sequencing the 16S rRNA gene in metagenomics
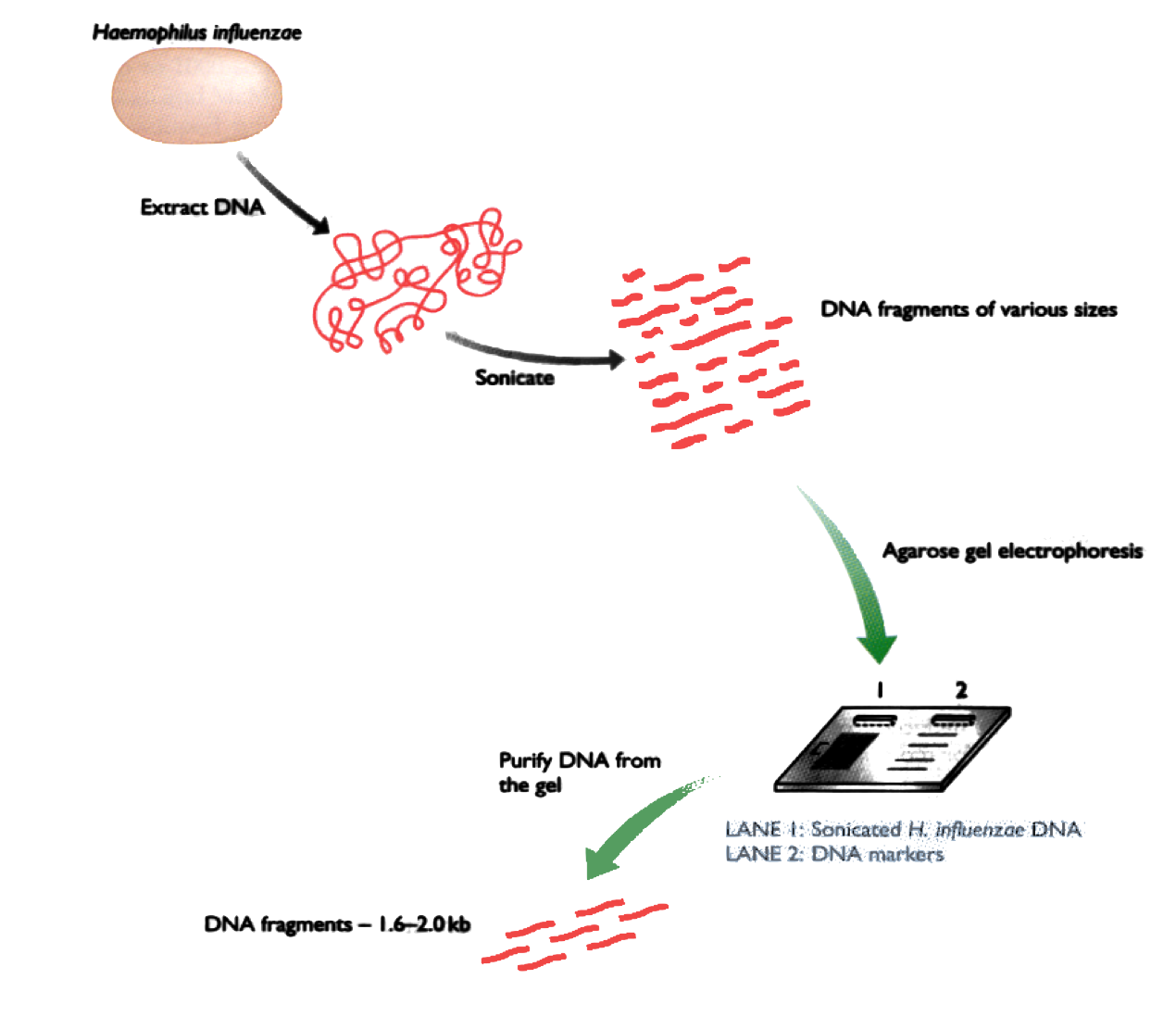
Shotgun DNA Sequencing
RNA sequencing
- Reverse transcription into cDNA
- Easier when there is a poly-A tail
- Usually the genome is known, and we care only about measuring gene expression
- If the genome is unknown, the mRNA gives us a good part of the genome
- That strategy is called expressed sequence tags (EST)
Fragments v/s reads
In all these cases we get a DNA fragment, about a few thousands bp long (let’s say 500–5000bp)
Each fragment is a physical object
Each strand can be sequenced and produce a read
Usually we have two reads for each fragment
They are sometimes called “forward” and “reverse” reads
How sequencing works
DNA sequencing
There are many sequencing methods, based on different physicochemical properties
- activity of restriction enzymes
- florescence
- electronic properties of nucleotides
- chemistry of DNA duplex formation
They are in constant development. We will focus on their key properties
See DNA sequencing on the WikiPedia for more details
Broadly speaking
- Sanger method: few short reads
- NGS: many very short reads
- 3rd generation: few long reads
Idea of Sanger method
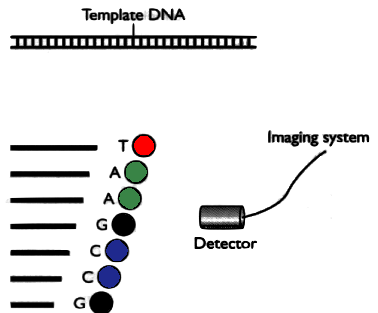
DNA is separated in 4 parts, each part is mixed with a different restriction enzyme. Then the fragments are separated by electrophoresis.
In a second version, each part is marked with a different fluorophores and then placed in a single capilar electrophoresis, with a light detector
Sanger requires cloning

Cloning vector
- know restriction-enzyme cut sites
- known binding-sites for primers
- antibiotic-resistance genes
- replication origin site
Part of the vector will be sequenced in every read
Sanger method’s result
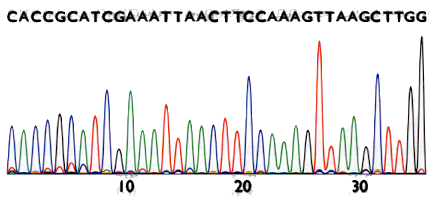
The result is a chromatogram. Filenames ending in .AB1 or .SCF
New generation sequencing
- Roche 454
- Illumina Solexa
- Ion Torrent
- ABI SOLID
Third generation
- DNA nanoball
- Pacific Biotech (PacBio)
Roche 454
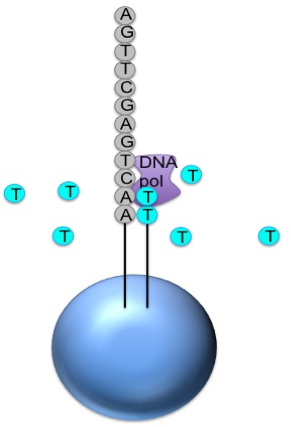
Generic adaptors are added to the ends and annealed to beads, one DNA fragment per bead
The fragments are then amplified by PCR
Each bead is placed in a single well of a slide
Each well will contain a single bead, covered in many PCR copies of a single sequence
The slide is flooded with one of the four NTP species
Roche 454
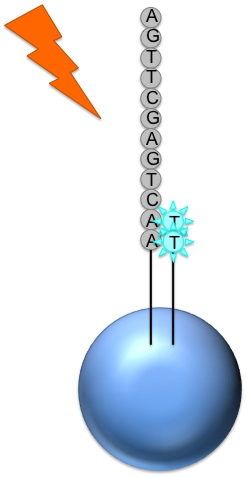
The nucleotide is incorporated when it matches the template
If that single base repeats, then more will be added
The addition of each nucleotide releases a light signal, and is registered with a hight resolution video
Roche 454
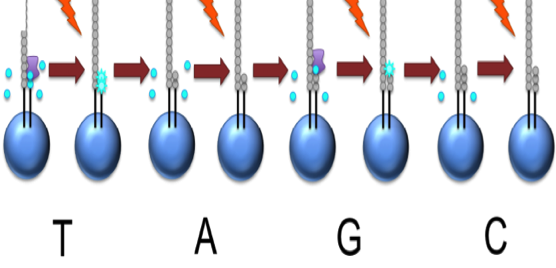
This NTP mix is washed away
The next NTP mix is now added and the process repeated, cycling through the four NTPs.
This kind of sequencing generates graphs for each sequence read, showing the signal density for each nucleotide wash
The sequence can then be determined computationally from the signal density in each wash.
Roche 454
Sequencing by synthesis
can sequence 0.7 gigabase per run
run time 23 hours
read length 700-800 bases
Illumina Solexa
also sequencing by synthesis
- old model Genome Analyzer can sequence 1Gb per run
- Solexa model can sequence 600 Gb per run
- HiSeq model 100 bases read length average 30Gb per run
- 11 day in regular mode
- 2 day in rapid run mode
Illumina Solexa
over 70% of the market
- ∼$1000 MiSeq
- ∼$3000 HighSeq
- ∼ 1.5 days
Illumina Solexa
- output of sequencing data per run: 600 Gb
- read lengths: approximately 100 bp
- cost is cheap
- run times are long: 3-10 days
ABI-SOLID
Sequencing by ligation
Supported Oligonucleotide Ligation and Detection (SOLiD)
- Solid 4 model can read 80-100 Gb per run (coverage 30Gb)
- average read length 50 bases
- fragment length 400-600-bp
ABI-SOLID
The advantage of this method is accuracy with each base interrogated twice
Major disadvantages
- short read lengths (50–75 bp)
- very long run times of 7 to 14 days
- need for computational infrastructure and expert computing personnel for analysis of the raw data
Ion torrent
- Developed by the inventors of 454 sequencing
- With two major changes.
- nucleotide sequences are detected electronically by changes in the pH of the surrounding solution rather than by the generation of light
- sequencing reaction is performed in a microchip with flow cells and electronic sensors on each cell
- The incorporated nucleotide is converted to an electronic signal
PacBio
- PacBio model RS II produces average read lengths over 10 kb, with an N50 of more than 20 kb and maximum read lengths over 60 kb
- Error rate of a continuous long read is relatively high (around 11%–15%)
- The error rate can be reduced by generating circular consensus sequences reads with sufficient sequencing passes.
Summary
| Method | Read length (bp) | Error rate (%) | No. of reads per run | Time per run | Cost per million bases (USD) |
|---|---|---|---|---|---|
| Sanger ABI 3730x1 | 600-1000 | 0.001 | 96 | 0.5–3 h | 500 |
| Illumina HiSeq 2500 | 2 × 250 | 0.1 | 1.2 × 109 (pairs) | 1–6 days | 0.04 |
| PacBio RS II: P6-C4 | 1.0–1.5×104 | 13 | 3.5–7.5 × 104 | 0.5–4 h | 0.4–0.8 |
| Oxford Nanopore MinION | 2–5 × 103 | 38 | 1.1–4.7 × 104 | 50 h | 6.44–17.9 |