Class 14: DNA Melting Temperature
Bioinformatics
Andrés Aravena
November 29, 2021
Polymerase Chain Reaction
Polymerase Chain Reaction
- Developed by Kary Mullis in the 1980s
- used to synthesize millions of copies of a given DNA sequence
PCR is based on
- Separate double-strand DNA using high temperatures, and
- Complementing each DNA single-strand using a polymerase that works at these high temperatures
Taq DNA polymerase
Isolated from Thermus aquaticus
Can polymerize deoxynucleotide precursors (dNTPs) in a temperature range of 75-80°C
The polymerase extends a pre-existing pairing, initially made by primers that bind spontaneously to specific sites on the DNA
A typical PCR reaction
A series of thermic cycles involving
- template DNA denaturation,
- primer annealing,
- and extension of the annealed primers by DNA polymerase
This three-step process is repeated 25-30 times
Result
Exponential accumulation of a specific fragment
Ends defined by the 5’ ends of primers
PCR reaction steps
They are repeated 20-30 times
- Denaturation of target DNA by heating to 90-95°C
- Temperature is lowered to about 5°C below primers melting temperature
- assuring specificity of primer annealing
- assuring specificity of the product
- Raising temperature to 70-73°C
- optimal temperature for primer extension
Primers
Short DNA fragments with a defined sequence complementary to the target DNA that we want to detect and amplify
Each PCR assay requires the presence of template DNA, primers, nucleotides, and DNA polymerase
The primers in the reaction specify the exact DNA product to be amplified
Melting temperature
Melting temperature
The melting temperature (\(T_m\)) is the one in which half of the DNA molecules are matched and half are not matched
The \(T_m\) shows the transition from double helical to random coil formation
It corresponds to the midpoint of the spectroscopic absorbance shift
Measuring
The \(T_m\) values are most simply measured by following the absorbance at 260 mM as a function of the temperature of the DNA solution and noting the midpoint of the hyperchromic rise
Melting temperature depends on
- the DNA GC base content,
- the cation concentration of the buffer
- Salt concentration
- the DNA double strand length
Salt concentration
The \(T_m\) increases with higher concentrations of salt due to the stabilizing effects that cations have on DNA duplex formation
More cations bind to duplex DNA than to the single strands
Different cations may have different effects on \(T_m.\)
Salt concentration
Most \(T_m\) research is done using \(Na^+\) as the primary cation; from a \(T_m\) standpoint, sodium, and potassium are equivalent
Divalent cations (such as \(Mg^{++}\)) also increase \(T_m\) but their effects are smaller than monovalent cations
Oligonucleotide sequence
Sequences with a higher fraction of G-C base pairs have a higher \(T_m\) than do AT-rich sequences
However, the \(T_m\) of an oligo is not simply the sum of AT and GC base content
Base stacking interactions must also be taken into account such that the actual specific sequence must be known to accurately predict \(T_m\)
The effects of neighboring bases as contributed through base stacking are called “nearest neighbor effects”
Formulas for \(T_m\) calculation
Formulas for \(T_m\) Calculation
Different formulas have been developed, which can be classified into two groups:
- nucleotide composition
- position-dependent
Nucleotide composition-based formulas depend on the GC-content, the number of base pairs, and the salt concentration
Position-dependent methods depend on parameters such as enthalpy (\(ΔH^0\)), entropy (\(ΔS^0\)), and Gibbs free energy (\(ΔG^0\))
Wallace-Itakura Formula
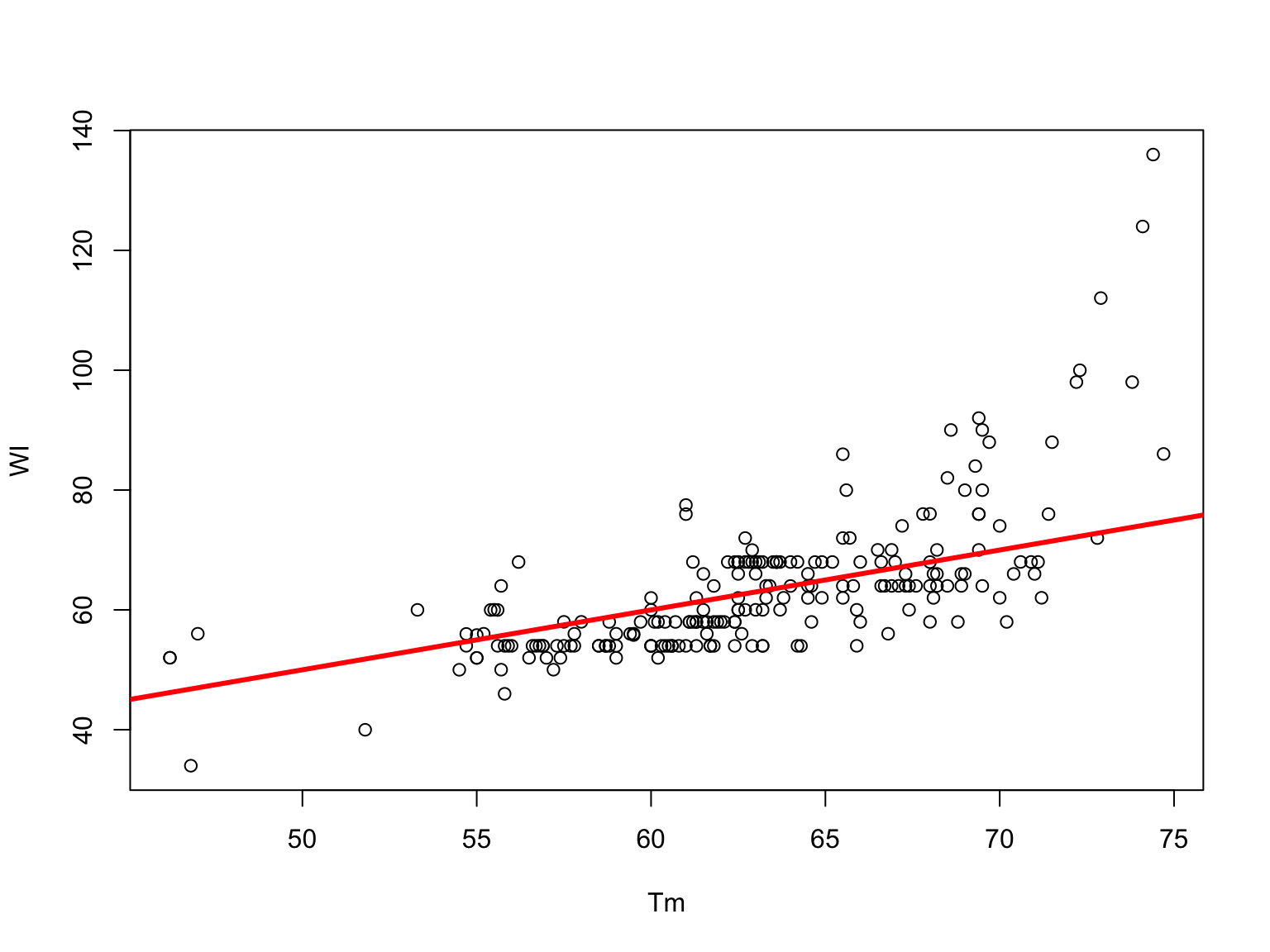
It is one of the simplest ones, but it may not give the exact result \[T_m = 4(G+C)+2(A+T)\] The formula was originally applied to the hybridization of probes in \(1 [mol/L]\) of \(NaCl\)
This rule overestimates the \(T_m\) of long duplexes
It gives reasonable results only in the range of 14-20 bp.
Wallace-Itakura v/s experimental \(T_m\)
Marmur and Doty Formula (1962)
The linear relation between the GC content and the \(T_m\) was determined using absorbance shift analysis
For a solvent containing 0.2 Molar of Na+, the melting temperature is \[T_m =69.3+0.41(\%GC)\] where \(T_m\) is in degrees Celsius
The measurement of the \(T_m\) is a way to determine the GC content of DNA
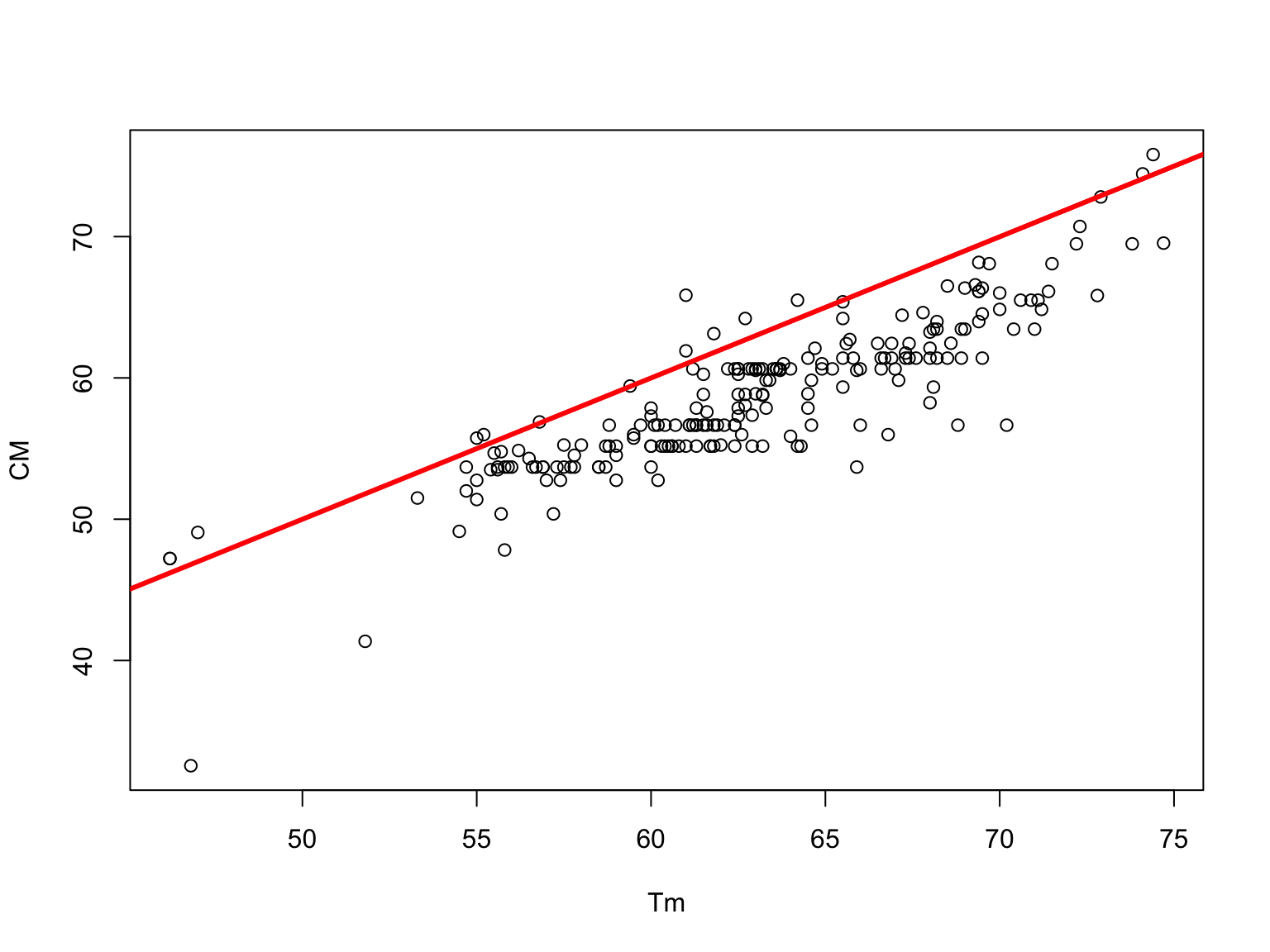
Chester and Marshak Formula (1992)
Chester and Marshak added a term to account for DNA strand length (n in base pairs) to estimate primer \(T_m\):
\[T_m =69.3+0.41(\%GC)-\frac{650}{n}\]
It is easy to see that if the DNA molecule is big (for example, if \(n>10^6\)), then this formula gives the same result as Marmur and Doty
Chester Marshak v/s experimental \(T_m\)
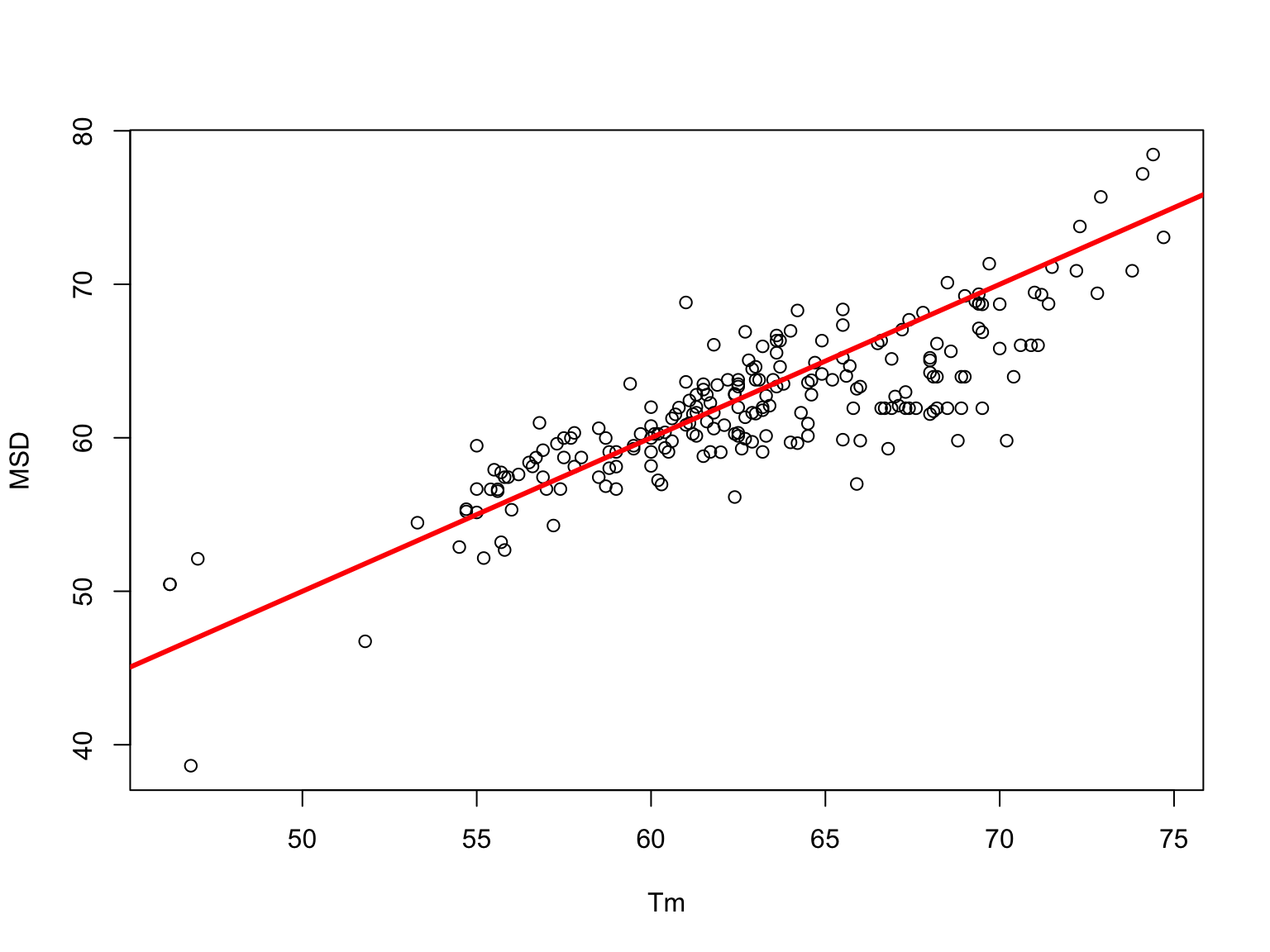
The Marmur-Schildkraut-Doty Equation (1964)
For ionic strength with a term for the \(Na^+\) concentration
\[T_m =81.5+16.6\log_{10}([Na^+])+0.41(\%GC)-\frac{b}{n}\]
Values between 500 and 750 have been used for \(b\) (a value that may increase with the ionic strength). Usually, the value \(b=500\) is used.
Marmur-Schildkraut-Doty v/s experimental \(T_m\)
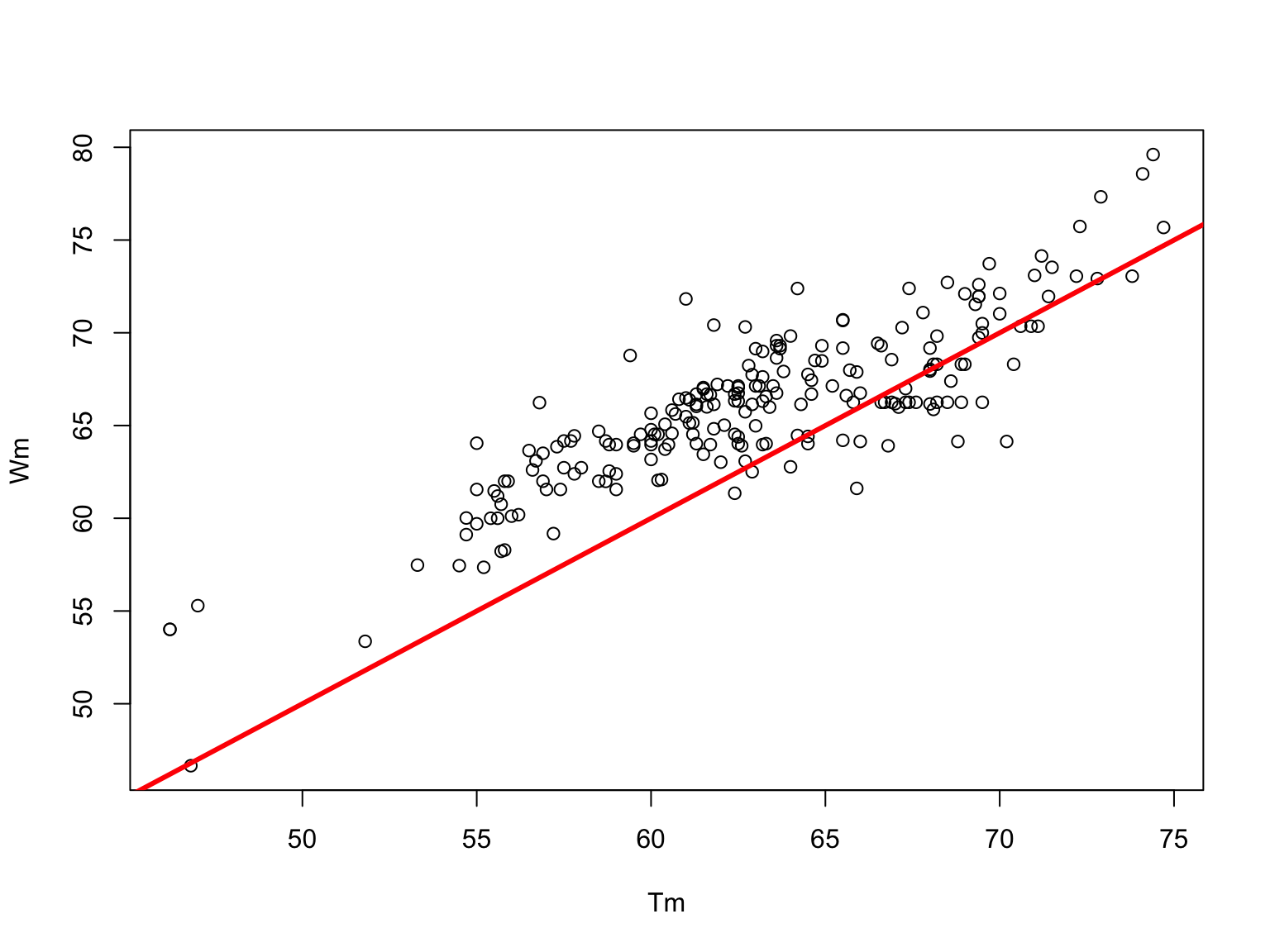
Wetmur Formula (1991)
\[\begin{aligned} T_m = & 81.5+16.6\log_{10}\left(\frac{[Na^+]}{1.0+0.7[Na^+]}\right) \\ & + 0.41(\%GC)-\frac{500}{n} \end{aligned}\]
This formula includes these variables with the salt concentration term modified to extend the range to 1 M \(Na^+\), a concentration routinely employed to maximize hybridization rates on blots. (Wetmur, 1991)
Wetmur prediction v/s experimental \(T_m\)
Position Dependent Calculation
These methods use thermodynamic parameters (entropy \(ΔS^0\), enthalpy \(ΔH^0\), and Gibbs free energy \(ΔG^0\)).
Experiments show that thermodynamic values for DNA melting do not depend only on base pair identity (A-T(U) or G-C)
Theoretical melting temperature is typically calculated assuming that the helix-coil transition is two-state
Two state model
SantaLucia, et al. suggest that the two-state model gives a reasonable approximation of melting temperature for duplexes with non-two-state transitions
\[\text{single-strand} + \text{single-strand} ⇆ \text{double-strand}\]
Thermodynamic Rule for \(T_m\)
For self-complementary oligonucleotide duplexes, \(T_m\) is calculated from the predicted \(ΔH^0\) and \(ΔS^0\), and the total oligonucleotide concentration \(C_T\), by using the equation \[T_m=\frac{ΔH^0}{ΔS^0+R\ln (C_T)}\] where \(R\) is the Boltzmann’s gas constant (1.987[cal/Kmol]) and temperature is measured in Kelvin degrees
(SantaLucia, 1998; Borer, Dengler, Tinoco, & Uhlenbeck, 1974)
Energy
\(ΔG^0\) is the free energy
Each \(ΔG^0\) term has enthalpic, \(ΔH^0\), and entropic, \(ΔS^0\) components
The \(ΔG^0_{37}\) can also be calculated from \(ΔH^0\) and \(ΔS^0\) parameters by using the equation: \[ΔG^0_T=ΔH^0(\text{total})-TΔS^0(\text{total})\] (SantaLucia, 1998)
Nearest-Neighbor Rule for energy
\[\begin{aligned} ΔG^0(\text{total})=∑_i n_iΔG^0(i)+ΔG^0(\text{init w/term G.C})\\ + ΔG^0(\text{init w/term A.T})+ΔG^0(\text{sym}) \end{aligned}\]
- \(ΔG^0(i)\) are the free-energy changes for the 10 possible Watson-Crick Nearest-Neighbors
- \(ΔG^0(1)=ΔG^0_{37}(AA/TT),\)
- \(ΔG^0(2)=ΔG^0_{37}(AT/TA),\) etc.
- \(n_i\) is the number of occurrences of each Nearest-Neighbor
- \(ΔG^0(sym)\) is \(+0.43\text{[k cal/mol]}\) if the duplex is self-complementary and zero if it is not
Formulas
\[\underbrace{\text{single-strand DNA}}_A + \underbrace{\text{primer}}_B ⇆ \underbrace{\text{double-strand DNA}}_{AB}\]
Equilibrium constant \(K\) \[K=\frac{[A][B]}{[AB]}=e^{-ΔG/RT}\] thus \[-RT\log K = ΔG\]
But we also have \[ΔG=ΔH - TΔS\] so \[TΔS-RT\log K = ΔH\] and therefore \[T = \frac{ΔH}{ΔS-R\log K}\]
Now \[K=\frac{([A]_{ini}-[AB])([B]_{ini}-[AB])}{[AB]}\]
Assuming that the initial concentration of primers is much larger than the initial DNA concentration, \([A]_{ini}<<[B]_{ini},\) and DNA will be the limiting factor. Thus, at the melting point we have \[[AB]=[A]_{ini}/2\] thus \[\begin{aligned} K&=\frac{([A]_{ini}-[A]_{ini}/2)([B]_{ini}-[A]_{ini}/2)}{[A]_{ini}/2}\\ &=\frac{[A]_{ini}/2⋅ ([B]_{ini}-[A]_{ini}/2)}{[A]_{ini}/2}\\ &=[B]_{ini}-[A]_{ini}/2 \end{aligned}\]
So, at the melting point, we have \[T_m = \frac{ΔH}{ΔS-R\log([B]_{ini}-[A]_{ini}/2)}\]
Since the initial DNA concentration is small, we have \[[B]_{ini}-[A]_{ini}/2 ≈ [B]_{ini}\] which gives us the final formula \[T_m = \frac{ΔH}{ΔS-R\log([B]_{ini})}\]